Bardo Climate Inc.
Transformative Bipolar Membranes
Bardo Climate Inc. is a spin-out from Vanderbilt University working to commercialize the bipolar membrane technology developed in Dr. Peter Pintauro's lab. Our goal is to scale-up and manufacture bipolar membranes with transformative performance and durability.
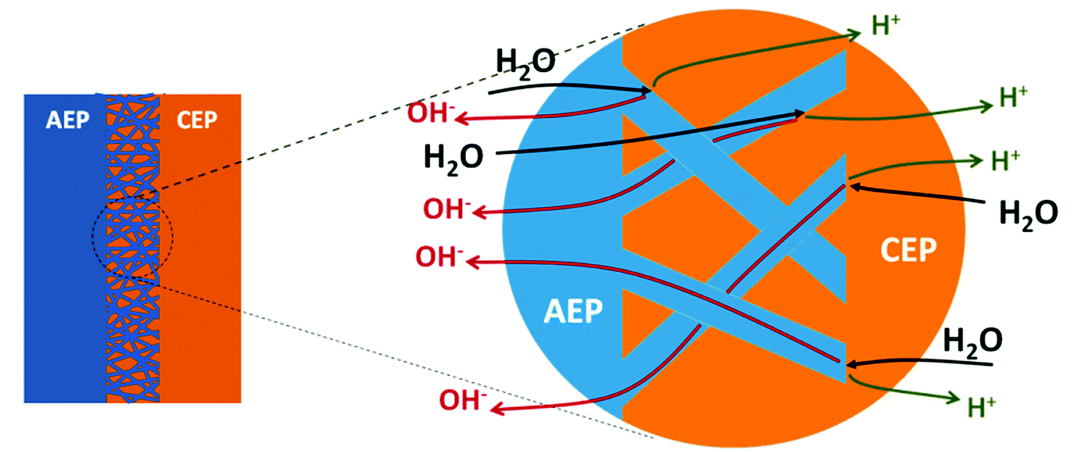
3D junction with interlocking AEP and CEP fibers
Example applications of the bipolar membrane (BPM)
Water Treatment
In water treatment, BPMs play a crucial role in desalination and selective ion separation, enabling the removal of unwanted salts and contaminants. They also help adjust pH levels without introducing chemical additives, making processes safer for the environment. BPMs can be especially valuable for wastewater recycling, helping industries achieve zero liquid discharge by recovering valuable by-products from brine streams.
Lithium Processing
BPMs are becoming increasingly important in lithium extraction and refining, a critical step in battery production for electric vehicles (conversion of lithium chloride to lithium hydroxide). This technology also allows for the recovery and recycling of acids and bases used in lithium processing.
Acid + Base Production
BPM electrodialysis can produce acids and bases (HCl, NaOH, etc.) more efficiently than conventional chlor-alkali electrolysis, reducing the environmental impact and operational costs for existing chemical supply.
Low cost acid and base production can also be used for ocean alkalinity enhancement or enhanced rock weathering, two approaches to carbon removal.
CO₂ Capture
BPMs can be used for electrodialytic carbon capture, which is one of the most promising approaches for capturing CO₂ from the atmosphere. To reach net-zero emissions by 2050, we need to capture and store around 5-10 gigatonnes of carbon dioxide (Gt-CO₂) per year through carbon capture technologies.
Additionally, bipolar membranes can support CO₂ electrolysis, where CO₂ is reduced to CO as a precursor to synthetic fuel production.
Long Duration Energy Storage
BPMs can be used in acid-base redox flow batteries, a type of battery technology that utilizes the difference in pH levels between acidic and basic solutions to store electrical energy. This is a promising option for long-duration energy storage applications due to the ability to store large amounts of energy with extremely low capacity cost, long-durations, and high scalability.
Hydrogen Electrolysis
BPMs contribute to energy-efficient water electrolysis by generating high-purity hydrogen and oxygen. Their unique ability to regulate pH in different compartments allows for optimized proton exchange and reduced membrane degradation, lowering energy requirements.
Advantages of the 3D Junction
Present-day BPMs, with a planar 2D junction (where the anion-exchange and cation-exchange membranes meet and where water is split into H⁺ and OH⁻) can only operate at current densities < 0.1 A/cm². Our membranes have a spun, 3D junction that enables 10x higher current densities and operation at 1 A/cm² without delamination or significant increases in voltage.
Meet the Team
Cyril Yee, CEO
Dr. Cyril Yee has held a number of positions in the cleantech and investment industries. Most recently, he was a Director at the Grantham Foundation for the Protection of the Environment leading investments in over 20 climate tech companies. He is the founder of Third Derivative, the world’s largest climate tech accelerator, and formerly led business development and strategy at INEOS Bio, a startup that built a $150M advanced biofuels plant. He has a Ph.D. in Chemistry from the Massachusetts Institute of Technology.
Peter Pintauro, CTO
Dr. Peter Pintauro was the H. Eugene McBrayer Professor Emeritus of Chemical Engineering in the Department of Chemical and Biomolecular Engineering at Vanderbilt University. Dr. Pintauro’s research interests are in the areas of electrochemical engineering, fuel cells and batteries, membrane science, and nanofiber electrospinning. He is a Fellow of the Electrochemical Society and the AICHE and a past President of the North American Membrane Society. He received a Ph.D. in Chemical Engineering from the University of California, Los Angeles.
Sam Lefkofsky, COO
Sam Lefkofsky leads operations at Bardo. In this role, he covers logistics, strategic planning, business development, financial oversight, and more. In addition to co-founding Bardo, Sam works on climate tech venture investments at the Grantham Foundation for the Protection of the Environment. Before that, he was at Third Derivative, the world’s largest climate tech accelerator, diligencing and supporting startups working on carbon capture and industrial decarbonization. Sam has a BA in Mechanical Engineering from Dartmouth College.
Ryszard Wycisk, Director of Product Development
Dr. Ryszard Wycisk is a chemical engineer with extensive experience in polymer chemistry and the development of ion-exchange membranes and membrane separation processes. He was integral to developing the bipolar membranes in Professor's Pintauro's lab at Vanderbilt. Prior to joining Bardo, Dr. Wycisk was the Director of Product Development at eSpin Technologies, a global leader in nanofiber-based filter media production. He has a Ph.D. in Chemical Engineering from the Wroclaw University of Science and Technology.
Allison Crow, Senior Chemical Engineer
Allison Crow is a chemical engineer with broad experience designing for the clean energy transition. Allison is completing her Ph.D. at the University of Colorado Boulder at the National Renewable Energy Laboratory (NREL) where her research focuses on synthesizing advanced BPMs, characterizing them with electrochemical techniques, and applying principles of electrochemical engineering to test their performance in BPMED stacks for the scale up of carbon capture and conversion technologies. Prior to her Ph.D. work at NREL, Allison worked on the global deployment of electric vehicles through large data analytics and stakeholder engagement at Rocky Mountain Institute. Allison received a BS in Chemical Engineering from Stanford University in 2016 and a MS in Chemical Engineering from the University of Colorado Boulder in 2021.
Mike McGrath, Senior Chemical Engineer
Mike McGrath is a chemical engineer with experience in developing ion exchange membrane materials. He can synthesize polymers, cast membranes, and build rigs to test membranes. He also has experience in project management of small startup teams. Prior to joining Bardo, he was lead engineer at Permeance Limited, a company fabricating gas separation membranes for carbon capture applications. Mike received a BS in Chemical Engineering from the University of Virginia and a PhD from the University of Colorado Boulder in Rich Noble’s membrane-focused research group.